
- Synthesis of platinum-cerium catalyst that works stably at high temperatures of 700 degrees or more
- An atomic catalyst that can greatly improve the performance of solid oxide fuel cells has been developed.
Dr. Kyung-jung Yoon and Ji-soo Shin of the Korea Institute of Science and Technology (KIST) Energy Materials Research Team announced that through joint research with Professor Yoon-Jung Lee of the Department of Energy Engineering at Hanyang University, they developed a single-atomic catalyst that stably operates even at high temperatures above 700 degrees while using only a small amount of platinum.
By allowing each platinum atom to react as a unit of a single atom without agglomeration at high temperatures, the platinum catalyst, which has been used only in low temperature fuel cells such as hydrogen electric vehicles, can be applied to fuel cell power generation systems operating at high temperatures.
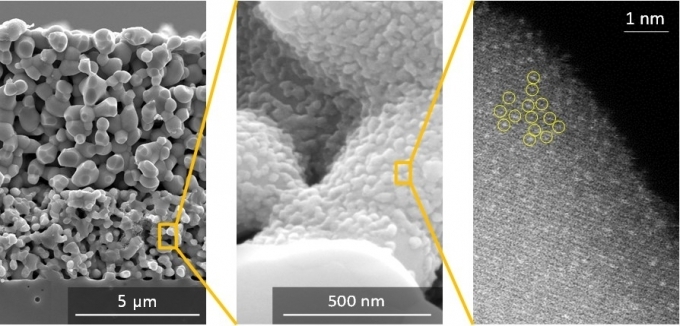
(Left) Solid oxide fuel cell electrode. (Center) A single atom catalyst formed on the inner surface of the electrode. (Right) Platinum atoms dispersed on the catalyst surface (bright dot) [KIST]
There are various types of fuel cells depending on the driving temperature and electrolyte. Among them, one of the most active fields of research worldwide is the solid oxide fuel cell using ceramic as an electrolyte.
Since the solid oxide fuel cell (SOFC) operates at a high temperature of 700°C or higher, it can achieve the highest efficiency among fuel cells, and even combined power generation to reproduce hydrogen by decomposing water vapor generated during the power generation process is possible. However, the key to commercialization is to develop a catalyst that can stably operate even at high temperatures.
Currently, platinum-based catalysts, which are widely used in the fuel cell field, exhibit superior performance that cannot be compared with any other material. However, at high temperatures, atoms are easily aggregated and structurally unstable, so they are not practically utilized.
Recently, single atom catalysts in which all atoms are individually dispersed are in the spotlight.If platinum atoms are individually dispersed and all atoms participate in the electrode reaction, the utilization of the catalyst is maximized, resulting in a great effect with very small amounts of platinum. Can be obtained. However, as the temperature rises, single atoms are easily aggregated. So far, single atom catalysts have been used only at low temperatures such as hydrogen electric vehicles, and there is no example applied to solid oxide fuel cells operating at high temperatures.
The KIST research team has developed a technology that allows platinum atoms to be uniformly fixed to the surface of the cerium oxide particles at about 1 nanometer intervals inside the electrode of a solid oxide fuel cell by strongly bonding platinum atoms and cerium (Ce) oxide nanoparticles. did.
Each platinum atom is individually dispersed on the surface of the cerium oxide nanoparticles and maintains the dispersed atomic state for a long time even at high temperature with strong bonding force, so all platinum atoms can participate smoothly in the reaction.
The research team found that this catalyst increased the reaction rate of the electrode by more than 10 times, and it was found to operate stably for more than 500 hours even at high temperatures of 700 degrees or higher, improving power and hydrogen production performance by 3 to 4 times.
In addition, a solution containing platinum and cerium ions, which are precursors of a single atom catalyst, is injected into the electrode of the fuel cell, and the catalyst is synthesized while the fuel cell is operating at high temperature. It is expected that it can be easily applied to existing fuel cells.
The research team said, “It is expected that the reaction speed of the electrode can be greatly improved while minimizing the amount of platinum used, which will accelerate the commercialization of the next-generation eco-friendly fuel cell, the solid oxide fuel cell.” “The development of process technology that can form atomic catalysts and mass synthesis technology must be developed.”
Dr. Kyungjoong Yoon said, “The catalyst developed in this study will be widely applicable to various types of solid oxide fuel cells and high-temperature electrochemical devices using an easy, simple and inexpensive process.” It is expected that the scope of application can be greatly expanded in the future to high-temperature thermochemical reactions and high-temperature electrochemical reactions, etc., as it suggests the possibility that it can operate stably in the future.”
This research was carried out as a major project of KIST and a development project for climate change response technology by the National Research Foundation of Korea, and the research results were published in the latest issue of’Energy & Environmental Science’. electrochemical devices
Read the most up to date Fuel Cell and Hydrogen Industry news at FuelCellsWorks




