Technologies
Solid Oxide Fuel Cell
Solid oxide fuel cells work at very high temperatures, the highest of all the fuel cell types at around 800ºC to 1,000°C. They can have efficiencies of over 60% when converting fuel to electricity; if the heat they produced is also harnessed; their overall efficiency in converting fuel to energy can be over 80%.
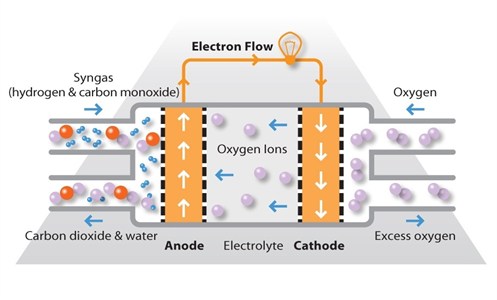
SOFCs use a solid ceramic electrolyte, such as zirconium oxide stabilized with yttrium oxide, instead of a liquid or membrane. Their high operating temperature means that fuels can be reformed within the fuel cell itself, eliminating the need for external reforming and allowing the units to be used with a variety of hydrocarbon fuels. They are also relatively resistant to small quantities of sulfur in the fuel, compared to other types of fuel cell, and can hence be used with coal gas.
A further advantage of the high operating temperature is that the reaction kinetics are improved, removing the need for a metal catalyst. There are however some disadvantages to the high temperature: these cells take longer to start up and reach operating temperature, they must be constructed of robust, heat-resistant materials, and they must be shielded to prevent heat loss.
There are three different SOFC geometries of SOFC: planar, coplanar and micro-tubular. In the planar design, components are assembled in flat stacks where the air and hydrogen traditionally flow through the unit via channels built into the anode and cathode. In the tubular design, air is supplied to the inside of an extended solid oxide tube (which is sealed at one end) while fuel flows around the outside of the tube. The tube itself forms the cathode and the cell components are constructed in layers around the tube.
SOFCs are used extensively in large and small stationary power generation: planar types find application in, for example, Bloom Energy’s 100 kW off-grid power generators and SOFCs with an output of a few kilowatts are being tested for smaller cogeneration applications, such as domestic combined heat and power (CHP). Micro-tubular SOFCs with output in the watt range are also being developed for small portable chargers.
Other types of fuel cell technologies available:









