Technologies
Alkaline Fuel Cell
Alkaline fuel cells (AFCs) were one of the first fuel cell technologies to be developed and were originally used by NASA in the space programme to produce both electricity and water aboard spacecraft. AFCs continued to be used on NASA space shuttles throughout the programme, alongside a limited number of commercial applications.
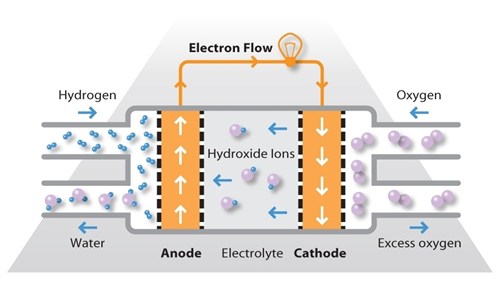
AFCs use an alkaline electrolyte such as potassium hydroxide in water and are generally fuelled with pure hydrogen. The first AFCs operated at between 100ºC and 250ºC but typical operating temperatures are now around 70ºC. As a result of the low operating temperature, it is not necessary to employ a platinum catalyst in the system and instead, a variety of non-precious metals can be used as catalysts to speed up the reactions occurring at the anode and cathode. Nickel is the most commonly used catalyst in AFC units.
Due to the rate at which the chemical reactions take place these cells offer relatively high fuel to electricity conversion efficiencies, as high as 60% in some applications.
Other types of fuel cell technologies available:









