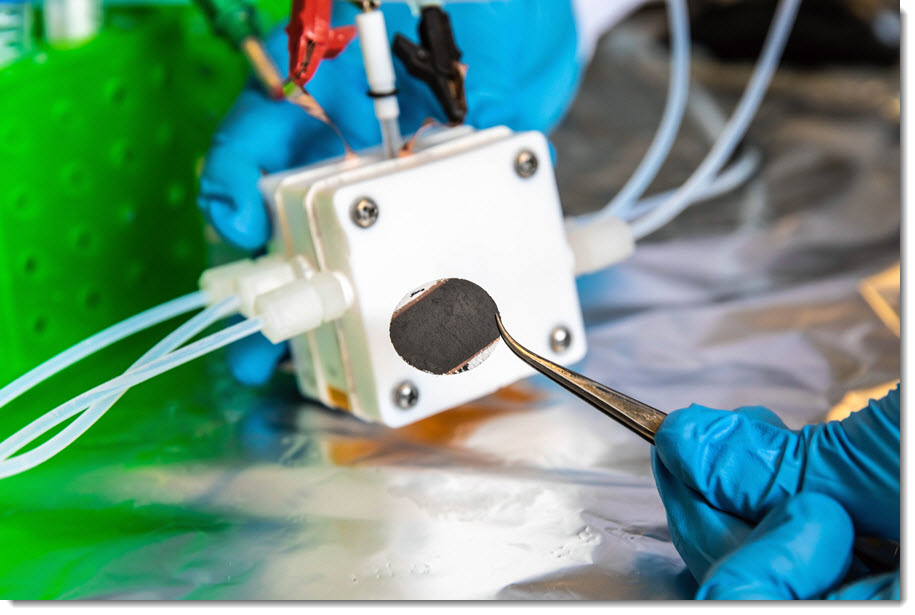
It is now possible to produce ’hydrogen’, a future energy source, more efficiently. This is because a catalyst has been developed to accelerate the reaction of water ’electrolysis’, a hydrogen production process.
A research team led by Professor Kwang S. Kim (National Scientist) in the Department of Chemistry at UNIST (President Lee Yong-Hoon) has developed a “metal organic compound” catalyst that will improve water electrolysis efficiency through theoretical calculations. This catalyst, which can be used in basic electrolytes, promotes the’oxygen-generating reaction’, which is referred to as a’bottleneck’ in’water electrolysis technology’, thereby increasing the overall reaction efficiency.
‘Water electrolysis’ is a method of decomposing water (H2O) by electricity to produce hydrogen (H2) and oxygen (O2). Here, two reactions of hydrogen and oxygen production occur simultaneously, and the problem is that the entire reaction proceeds in response to the slow “oxygen-generating reaction”. Therefore, the slower the oxygen generating reaction, the slower the hydrogen production rate and the hydrogen production rate directly connected to it.
The research team proposed a new solution with a catalyst developed using a metal organic framework (MOF) containing nickel and iron. The metal organic skeleton is a material in which a metal and an organic material form a framework like a’reinforcing bar’ of a building. There are many fine-sized pores (channels), so the surface area is large, and the metal atom where the catalytic reaction occurs is exposed to the surface. Moreover, compared to iridium (Ir) used in commercial catalysts, nickel and iron have more reserves and are cheaper.
The structure of this catalyst has been designed via theoretical calculations by Miran Ha in the Doctoral program of Natual Sciences at UNIST, the co-author of the study. Through the simulation, the optimal structure and composition of the metal organism skeleton were found. “Doping iron on the nickel metal organic framework improves the reactivity at the point of a single atom and improves overall reactivity,” she noted.
The catalyst developed this time can produce a large amount of hydrogen (current density) with much less energy (overvoltage) than conventional iridium oxide catalysts. As a result of evaluating the performance of the actual catalyst by making an’alkali anion exchange membrane water electrolysis device’, a current density of 0.5 A (ampere) per unit area (cm2) was achieved at a voltage of 300 mV (millivolt). This is a value sufficient for commercial use of the catalyst. It also showed excellent durability when operated for over 1,000 hours.
“This study not only improved the slow’oxygen-generating reaction’ rate problem, but also solved the price and stability problems of existing commercial catalysts at the same time,” says Professor Kim. “We expect that the newly-developed catalyst can be used in various energy conversion devices.”
Journal Reference
Pandiarajan Thangavel, Miran Ha, Shanmugasundaram Kumaraguru, et al., “aphene-nanoplatelets-supported NiFe-MOF: high-efficiency and ultra-stable oxygen electrodes for sustained alkaline anion exchange membrane water electrolysis,” Energy Environ. Sci., (2020).
Source: UNIST
Read the most up to date Fuel Cell and Hydrogen Industry news at FuelCellsWorks




