
A research team led by Shinshu University’s Tsuyoshi Takata, Takashi Hisatomi and Kazunari Domen succeeded in developing a photocatalyst that can split water into hydrogen and oxygen at a quantum efficiency close to 100%.
The team consisted of their colleagues from Yamaguchi University, The University of Tokyo and National Institute of Advanced Industrial Science and Technology (AIST).
The team produced an ideal photocatalyst structure composed of semiconductor particles and cocatalysts. H2 and O2 evolution cocatalysts were selectively photodeposited on different facets of crystalline SrTiO3(Al-doped) particles due to anisotropic charge transport. This photocatalyst structure effectively prevented charge recombination losses, reaching the upper limit of quantum efficiency.
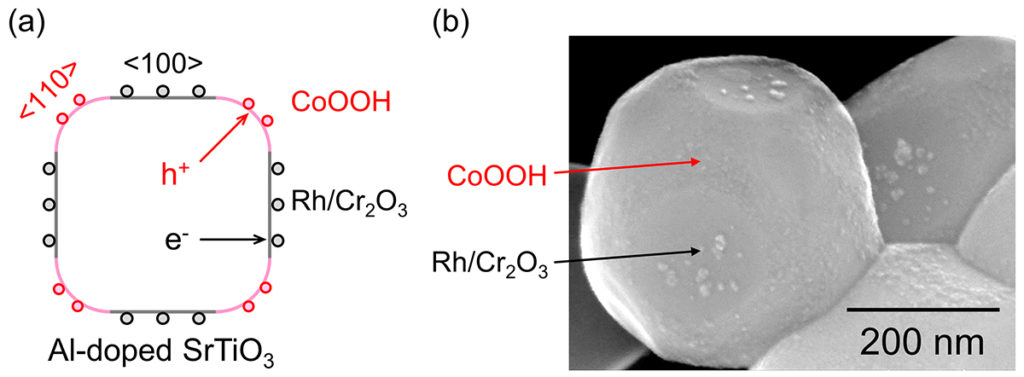
Figure 1 – Schematic structure (a) and scanning electron microscope image (b) of Al-doped SrTiO3 site-selectively coloaded with a hydrogen evolution cocatalyst (Rh/Cr2O3) and an oxygen evolution cocatalyst (CoOOH).
Water splitting reaction driven by solar energy is a technology for producing renewable solar hydrogen on a large scale. To put such technology to practical use, the production cost of solar hydrogen must be significantly reduced [1]. This requires the reaction system that can split water efficiently and can be scaled up easily. A system consisting of particulate semiconductor photocatalysts can be expanded over a large area with relatively simple processes. Therefore, it will make great strides toward large-scale solar hydrogen production if photocatalysts driving the sunlight-driven water splitting reaction with high efficiency are developed.
To upgrade the solar energy conversion efficiency of photocatalytic water splitting, it is necessary to improve two factors: widening the wavelength range of light used by the photocatalyst for the reaction and increasing the quantum yield at each wavelength. The former is determined by the bandgap of the photocatalyst material used, and the latter is determined by the quality of the photocatalyst material and the functionality of the cocatalyst used to promote the reaction. However, photocatalytic water splitting is an endergonic reaction involving multi-electron transfer occurring in a non-equilibrium state.
This study refined the design and operating principle for advancing water splitting methods with a high quantum efficiency. The knowledge obtained in this study will propel the field of photocatalytic water splitting further to enable the scalable solar hydrogen production.
The project was made possible through the support of NEDO (New Energy and Industrial Technology Development Organization) under the “Artificial photosynthesis project”.
Title: Photocatalytic water splitting with a quantum efficiency of almost unity
Authors:Tsuyoshi Takata, Junzhe Jiang, Yoshihisa Sakata, Mamiko Nakabayashi, Naoya Shibata, Vikas Nandal, Kazuhiko Seki, Takashi Hisatomi, Kazunari Domen
Journal:Nature, 581, 411-414 (2020)
DOI10.1038/s41586-020-2278-9
Read the most up to date Fuel Cell and Hydrogen Industry news at FuelCellsWorks




