Among its many properties, platinum is highly sought after for its catalytic power. However, this metal has a high cost and its exploitation leaves a significant environmental footprint.
In order to use it in smaller quantities, or even to replace it, researchers from IRCER (CNRS / University of Limoges), IEM (CNRS / ENSCM / University of Montpellier) as well as from Brazil, Japan, from the United States, Turkey, and India, have developed an ultra-porous ceramic, which allows an extremely thin layer of platinum to fulfill its role as a catalyst in a fuel cell. Published in the journal Applied Catalysis B: Environmental, this work could be extended to reduce the use of platinum in other areas.
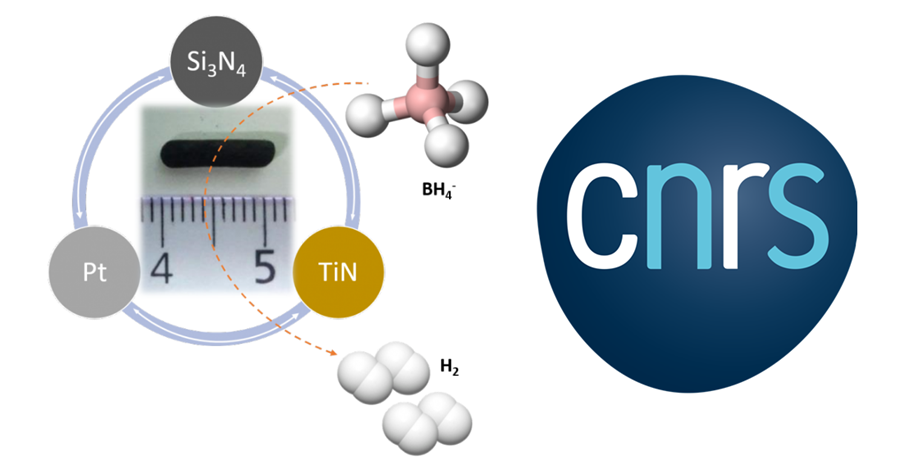
Dihydrogen is an energy carrier of interest due to its high energy density. It can be produced from a variety of sources, using reactions involving catalysts. Platinum is an essential element in most of these cases, but this metal is in short supply and is in high demand in jewelry and for medical implants. Considerable efforts are therefore being made to find solutions that limit or optimize their use. D ‘re scientists ceramics Research Institute (IRCER, CNRS / University of Limoges) and the European Membrane Institute (EMI, CNRS / ENSCM / University of Montpellier) and the Federal University of Santa Catarina (Brazil), Nagoya Institute of Technology (Japan), theNational Institute of Standards and Technologies (United States), the Middle East Technical University (Turkey) and the Indian Institute of Technology in Madras (India) have thus developed ultra-porous ceramics whose structure and the particular composition allow the use of very small amounts of platinum nanoparticles, highly dispersed and accessible. This robust system also has the advantage of being reusable.
The ceramic in question, produced by a so-called “precursor route” technique, is made from titanium, nitrogen, and silicon. When a thin layer of platinum is deposited on its surface, the metal is distributed in the pores of the material and thus finds itself with a multiplied action surface. The effect is then maximized thanks to the ceramic support, further increasing the capacity of the same quantity of platinum to catalyze the production of hydrogen. Scientists are now working on getting the complete ceramic/catalyst system in one step while replacing platinum with less expensive metals.
Reference
Abhijeet Lale, Maira Debarba Mallmann, Shotaro Tada, Alina Bruma, Saim Özkar, Ravi Kumar, Masaaki Haneda, Ricardo Antonio Francisco Machado, Yuji Iwamoto, Umit B. Demirci and Samuel Bernard. Highly active, robust and reusable micro- / mesoporous TiN / Si 3 N 4 nanocomposite-based catalysts for clean energy: Understanding the key role of TiN nanoclusters and amorphous Si 3 N 4 matrix in the performance of the catalyst system . Applied Catalysis B: Environmental , 2020, 272, 118975.
https://doi.org/10.1016/j.apcatb.2020.118975
Photo: A stable and efficient nanocomposite based on TiN / Si3N4 as a catalyst to boost the production of hydrogen from hydrides. © Samuel Bernard
Read the most up to date Fuel Cell and Hydrogen Industry news at FuelCellsWorks




